Galvanic corrosion is often a major concern anywhere pipes of dissimilar materials connect. I routinely see this visible in basements. It’s important to educate homeowners and prospective home buyers on how to prevent galvanic corrosion. A simple preventive repair can easily turn into very costly plumbing overhaul or flood clean-up.
I often find galvanized plumbing lines in homes built before 1960. Galvanized plumbing was introduced as a significant upgrade to previously-used lead pipes. However, galvanized plumbing is more likely to corrode than other materials. Galvanized plumbing has an expected life of 40-50 years. Pipes that are installed properly and are well-maintained can last significantly longer. If there is any sign of corrosion on the galvanized plumbing, replacement should be considered.
What is Galvanic Corrosion?
Galvanic corrosion or “bimetallic corrosion” is the accelerated corrosion of a metal. This happens because of an electrical contact (including physical contact) with a more noble metal or nonmetallic conductor (the cathode) in a corrosive electrolyte.
The less corrosion-resistant or the “active” member of the couple experiences accelerated corrosion. The more corrosion-resistant or the “noble” member of the couple experiences reduced corrosion due to the “cathodic protection” effect.
The most severe attack occurs at the joint between the two dissimilar metals. Further away from the bi-metallic joint, the degree of accelerated attack is reduced.
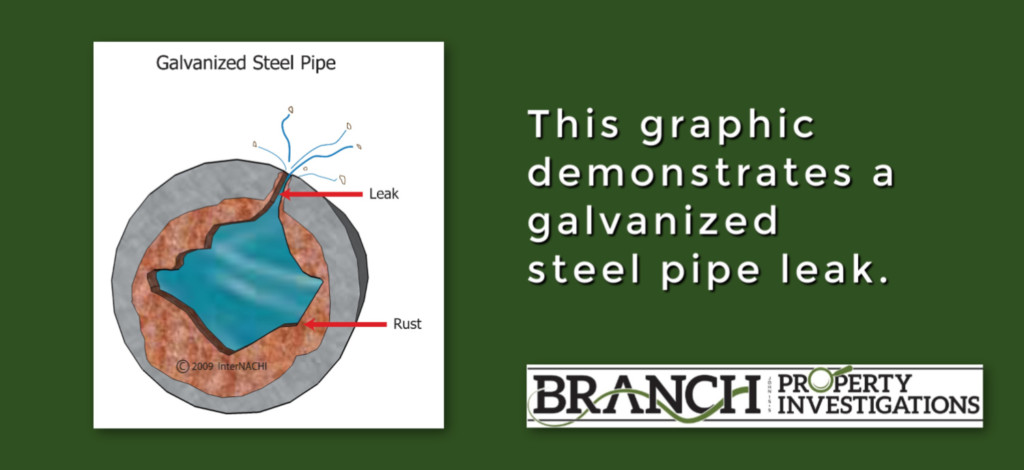
I have seen this most often where a galvanized steel plumbing line connects with a new copper line at a threaded connection. The corrosion looks like typical rust. Upon closer inspection, you can see the pipe is being eaten-through as the two dissimilar metals are in contact. In this case, the iron/steel is the more corrosive (less noble) metal and the copper is the more noble metal. The corrosion can lead to sudden pipe failure, flooding and a major mess — all of which are preventable.
A more subtle contributor to the presence of galvanic corrosion is the chemical makeup of the water that passes through the pipe. It can be an accelerant for the onset of galvanic corrosion.
MN Plumbing Code (2015)
As of 2015 in Minnesota, copper tubing can no longer be directly connected to galvanized steel pipes (605.17 Joint Between Various Materials). When dissimilar metals are connected to each other and water passes between them, a galvanic reaction can occur, causing the less noble metal to corrode.
I see connections between galvanized steel and copper routinely but they’re no longer allowed. To connect copper tubing to threaded pipe joints, there must now be a brass adapter, a 6″ brass nipple, a dielectric fitting, or a dielectric union used.
Steps to Prevent Galvanic Corrosion
The most common ways to prevent galvanic corrosion include:
- Electrically insulate the dissimilar metals using a brass nipple or other dielectric fitting (plastic in some applications) between the materials. A coat of grease and pipe tape can help to insulate steel and aluminum parts. That shouldn’t be the only protection.
- Shield the metal from ionic compounds. This is often accomplished by encasing the metal in epoxy or plastic. Coating or protection should be applied to the more noble of the two metals, if it is impossible to coat both. Otherwise, greatly accelerated corrosion may occur at points of imperfection in the less noble (more active or anodic) metal.
- Replace the iron or galvanized steel pipe with one of the same electric potential (such as copper to copper) or with plastic.
- Avoid threaded connections as they are most severely weakened by galvanic corrosion.
In summary, galvanic corrosion can occur in homes wherever dissimilar metals are connected in plumbing and/or gas lines without the proper method of connection. If you have a concern about this issue, please call us to schedule a Single-Item Home Inspection at (612) 440-8466.